Nearly 80% of data in Chinese clinical trials have been fabricated
Thanks to a subscriber for this article from biospectrumasia.com which may be of interest. Here is a section:
A recent investigation led by the Chinese State Food and Drug Administration (SFDA) revealed that nearly 80 percent of the data used in clinical trials of new pharmaceutical drugs have been "fabricated." The investigation looked at data from 1,622 clinical trials for new pharmaceutical drugs currently awaiting approval.
With these fraudulent activities coming to light nearly 80 percent of current drug applications, which were awaiting approval for mass production, have now been cancelled. The SFDA found that the more than 80 percent of the data failed to meet analysis requirements, were incomplete, or totally non-existent.
The lack of governance and perhaps more important integrity in the Chinese corporate sector is truly worrying from the perspective of consumers and particularly medical care customers. However as the above article goes on to highlight very few people are overly surprised at these findings since general expectations for supply chain and research integrity are so low.
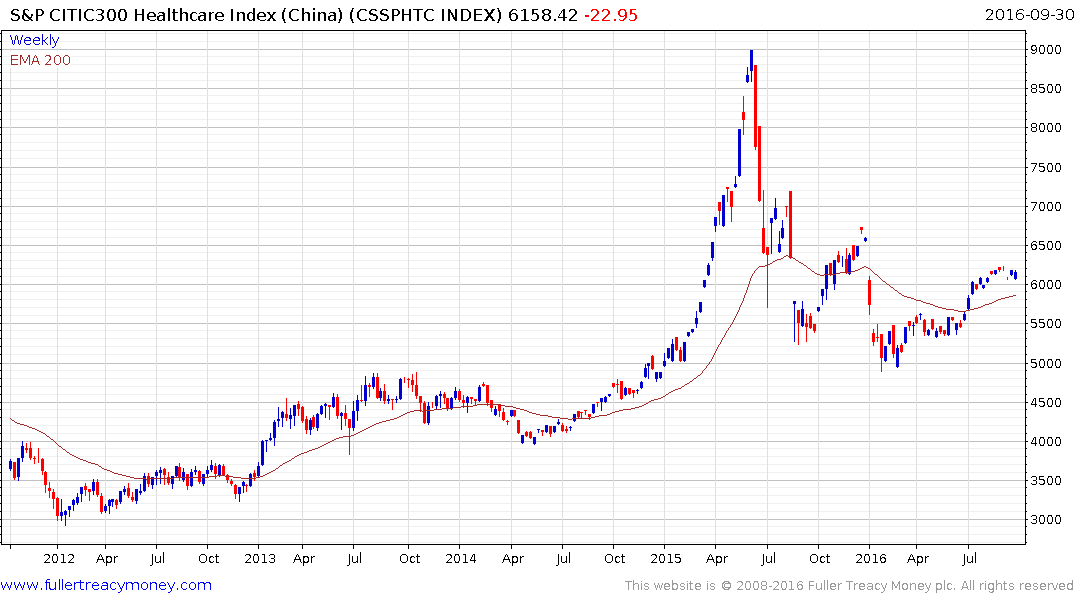
The mainland Chinese market is closed this week for the Mid-Autumn holiday but overall the S&P/Citic 300 Healthcare Index has been among the market’s better performers. The Index has been consolidating above the 200-day MA since July and a sustained move below it would be required to question medium-term scope for continued higher to lateral ranging.
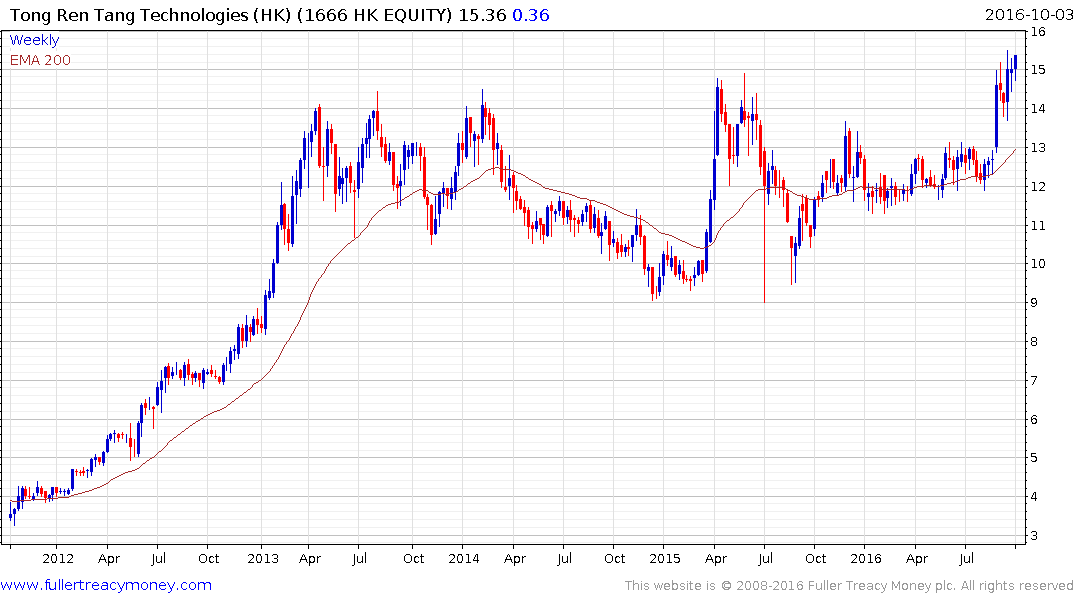
Larger more established companies like Tong Ren Tang Technologies appear to have been little affected by this news. The share broke out of an almost 4-year range two weeks ago and moved to a new closing high today. A sustained move below $15 would be required to question to question medium-term scope for additional upside.


