The FDA Wants a Covid-19 Vaccine That Really Works
This article by Max Nisen for Bloomberg may be of interest to subscribers. Here is a section:
The path the FDA outlines is a long one. It’s going to take a while to recruit and enroll 30,000 people in a trial and give half of them two shots in the arm — as Moderna Therapeutics Inc. intends to do to test its candidate. And until a sufficient number of subjects in the placebo arm of such a trial contract Covid-19, there won’t be any firm results. Any number of variables could cause further delays: bad luck, a poor vaccine performance, or slowing case growth.
The FDA is by no means ignoring the urgency of the moment. Its guidance includes a variety of concessions on safety data and other issues that are meant to speed the process. But the world can be grateful the agency is willing to bend only so far.
Setting a high standard for a vaccine that will be administered to hundreds of millions of people is imperative. It is the minimum requirement to instill faith in the population that it is worth accepting. I have every expectation that the advances in genetic sequencing and editing will deliver a positive result this year and that a true second wave will be avoided.
The impatience many people feel is perhaps the biggest obstacle to containing the spread before a vaccine has been delivered. I even find myself being less vigilant now than I was a few months ago. That is despite the massive swell of community spread and the greater likelihood of contracting it as a result. That’s a good example of how even the most extreme situation can assume an air of normalcy after a relatively short period of time. That’s one of humanity’s greatest survival instincts, although it is not especially helpful in the short term.
China is already rolling out a vaccine candidate in its armed forces. They appear to be taking the exact opposite approach to the FDA. Here is a section from an article from futurism.com
To date, trials of the vaccine have showed mixed results, boosting immune response in only some participants. About half the volunteers who took it had little immune response, STAT reported at the time. Some experts suggested the vaccine could be helpful for the most at-risk age groups.
The drug is a “viral vector vaccine,” meaning it contains a live but significantly weakened adenovirus 5 (Ad5) that has portions of the SARS-CoV-2 virus grafted onto it. In short, the adenovirus shows the immune system how to identify the coronavirus.
“This is the story of Ad5,” Kathryn Edwards, scientific director of the Vanderbilt Vaccine Research Program in Nashville, told STAT at the time. “It’s the concern with Ad5 that’s been there from the beginning: That if you have antibody to the vector, then you don’t get as good a [vaccine] take.”
Confidence that a shot will deliver immunity is essential to consumer confidence. Personally, I will be waiting for a well-researched solution with strong analytical data.
The stock market appears willing to look past short-term obstacles. A good part of the reason for that is because of the expectation of additional liquidity flows, interest rates that are likely to stay low for a long time and the wider yield differential stocks enjoy over bonds.
That yield spread is evident right across international markets but is much wider in markets outside the USA. Canada, Australia, New Zealand, Switzerland, the EU and UK all have wider equity yield - bond yield spreads.
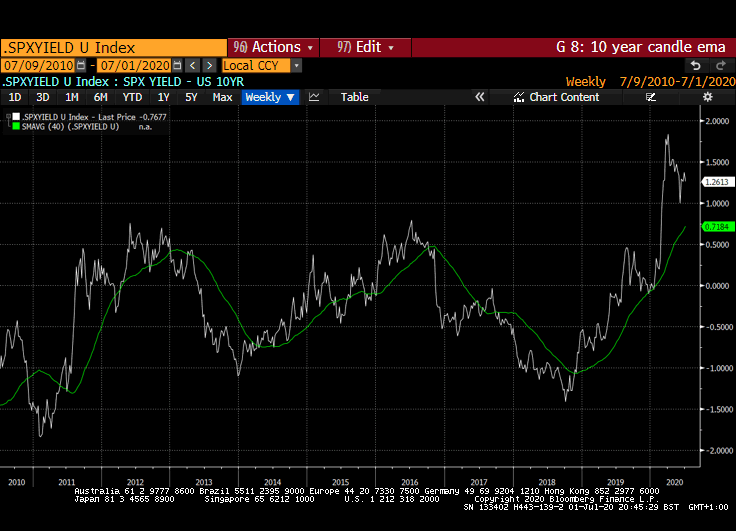
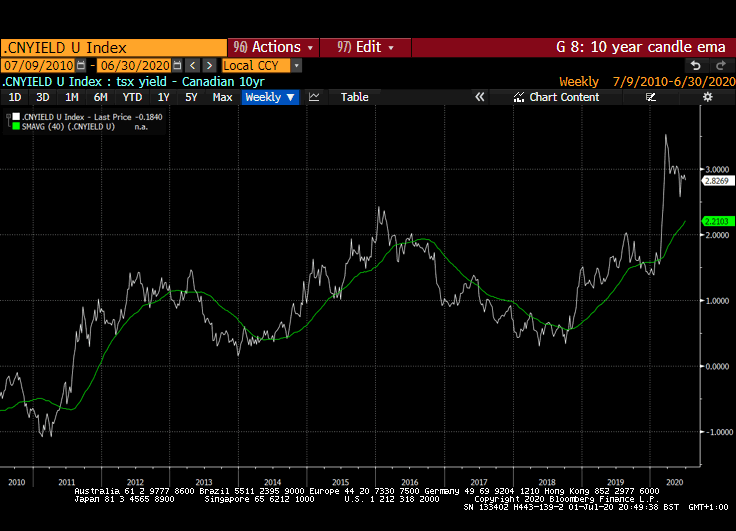
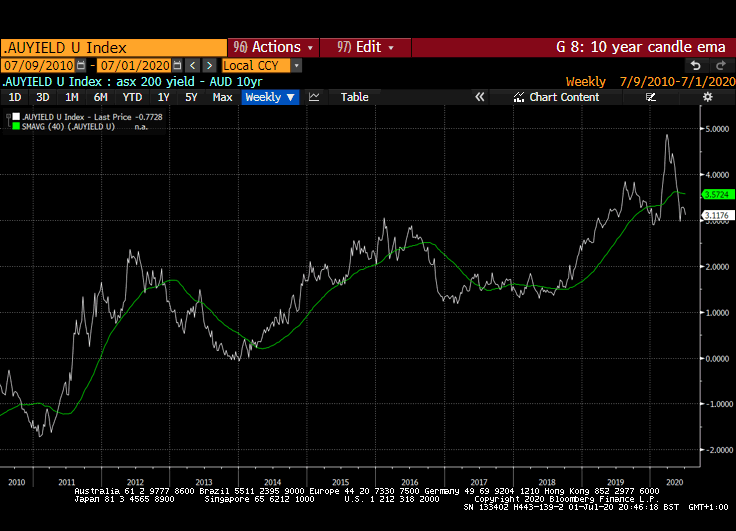
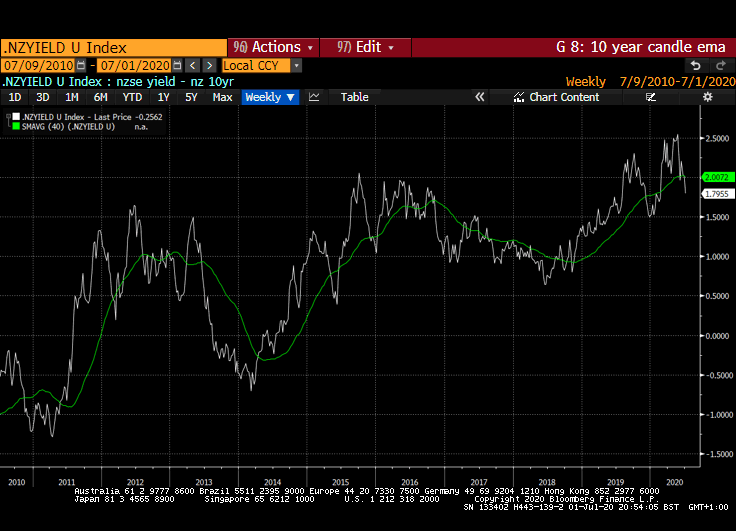
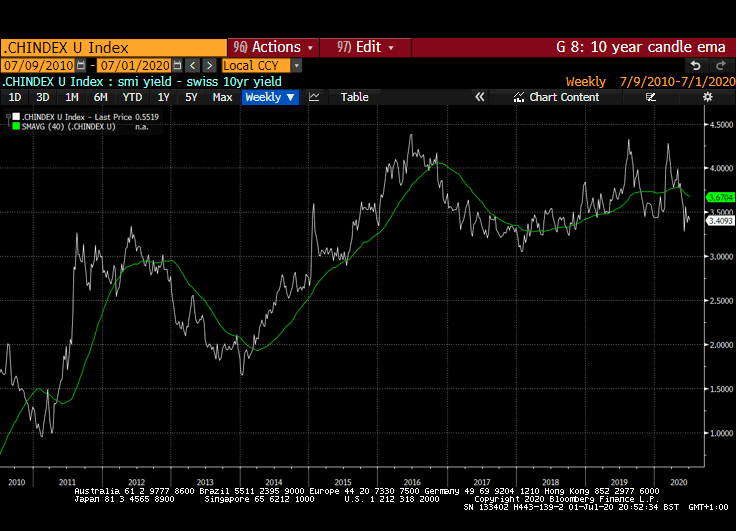
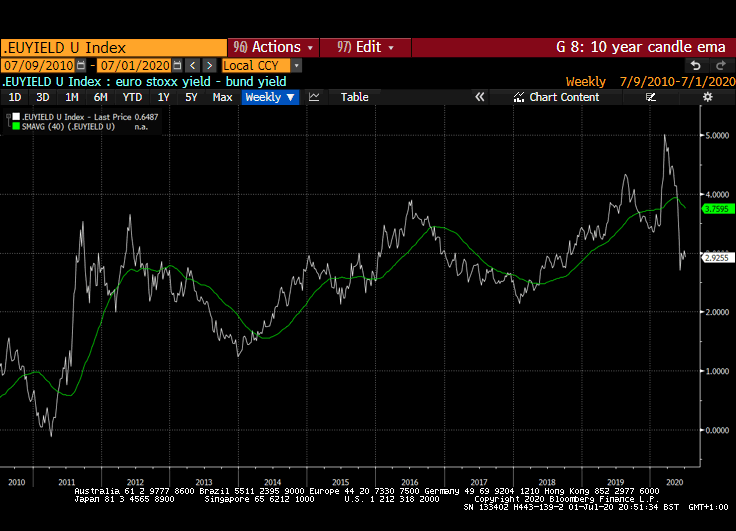
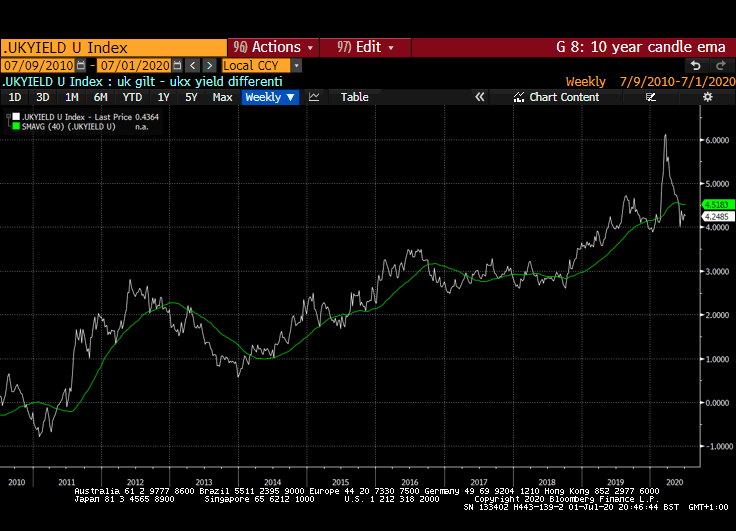
Meanwhile the Dollar continues to look susceptible to additional weakness. While the broad technology sector continues to contribute to US outperformance, the relative value represented by international markets is likely to continue to attract flows.


