Moderna kicks off Phase 1 trial of 3 different mRNA HIV vaccines
This article from NewAtlas may be of interest to subscribers. Here is a section:
“Finding an HIV vaccine has proven to be a daunting scientific challenge,” said NIAID director Anthony Fauci, in a statement announcing the Phase 1 trial. “With the success of safe and highly effective COVID-19 vaccines, we have an exciting opportunity to learn whether mRNA technology can achieve similar results against HIV infection.”
The Phase 1 trial will enroll around 100 healthy adults, with the initial goal of evaluating the safety and immune responses to three different mRNA vaccine formulations. Each subject will receive three doses of their assigned mRNA formulation over a six-month period.
In the same way mRNA COVID-19 vaccines are designed to train the immune system to respond to the spike protein on the surface of SARS-CoV-2, these experimental vaccines focus on the HIV equivalent of the spike protein antigen target, known as an envelope glycoprotein trimer.
This protein on the surface of HIV particles is much more complex that the coronavirus spike protein, so Moderna has developed three different mRNA formulations to test, each encoding for a slightly different protein architecture.
The trial is expected to run until mid-2023. By that point it is hoped one of the three formulations will have demonstrated robust immune responses and Phase 2 trials can commence.
This announcement holds out promise that an intransigent health issue can be addressed with a shot. It also highlights the fact that rushed permitting for COVID-19 vaccines is not about to be repeated. If the trials schedule discussed in the above article is followed, it will be a decade before a potential solution reaches market. It is also likely to be held to a much higher standard of proof.
The biotech sector became a winner-takes-all market for the duration of the pandemic. The vaccine inventors vied for market dominance with their market share. Traders favoured the solution which appeared to offer the most protection, as successive waves of variants reordered the attraction of each one.
Moderna quickly became the largest contributor to the performance of the biotech and IPO ETFs. The share collapsed as Pfizer’s vaccine gained more mass market appeal. Moderna is now chasing the “next big thing” but it will take long years of development work to bring products to market.
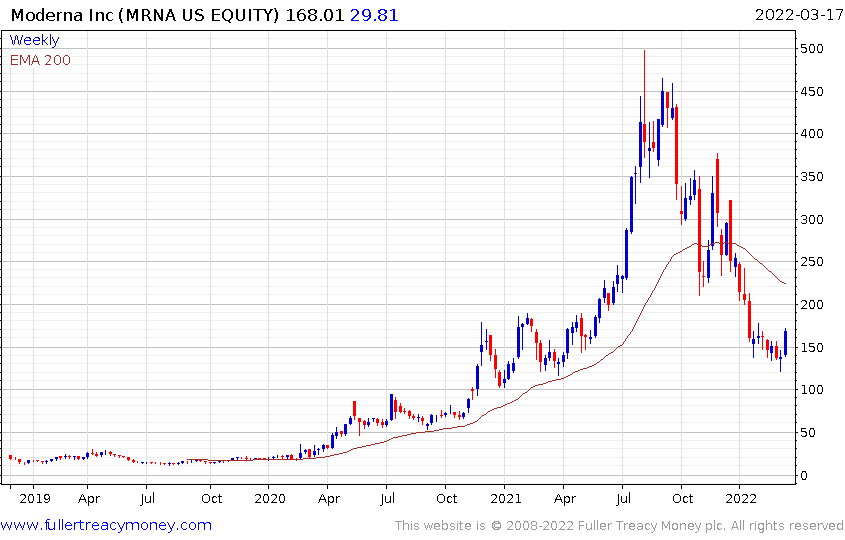
The share is now steadying from the region of the 1000-day MA.
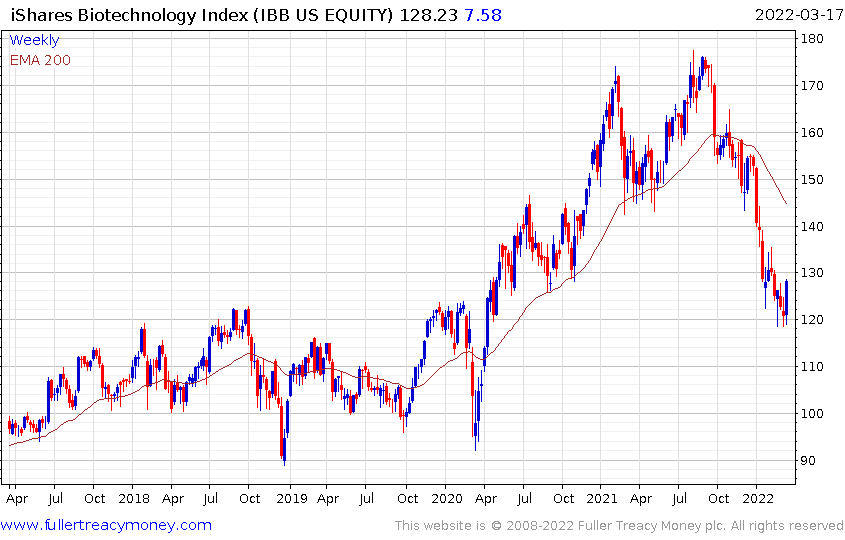
The Biotech ETF is showing initial signs of steadying in the region of the upper side of the underlying range.
The relative weighting on vaccine producers has been much reduced in the ETF. Amgen and Gilead are now the largest constituents and the breadth of work pursued in the sector is likely to be better reflected by its performance.
Back to top

